TB & Respiratory Infections
HAI & Other Infectious Diseases
STIs
Emerging Infectious Diseases
EN
FlashDetect™ LyocartD SARS-CoV-2&Flu A&Flu B Assay
FlashDetect™ LyocartE SARS-CoV-2&Flu A&Flu B Assay
Simultaneous detection of 3 respiratory infections in one cartridge:
SARS-CoV-2 (ORF1ab, N gene), Influenza A (M gene) and Influenza B (NS1 gene).
Results in 20-30 mins
LoD as low as 100 copies/mL (SARS-CoV-2)
Includes internal control
Pre-loaded with lyophilized reagents
Contamination-free
Room temperature storage and transportation

Product Name | FlashDetect™ LyocartD SARS-CoV-2&Flu A&Flu B Assay | FlashDetect™ LyocartE SARS-CoV-2&Flu A&Flu B Assay | ||
Method | Real-Time PCR All-in-one cartridge integrates sample prep and PCR amplification | |||
| Analytes | SARS-CoV-2 (ORF1ab gene, N gene), Influenza A (Flu A), Influenza B (Flu B), Internal Control (RNase P gene) | |||
| LoD | 200 copies/mL(SARS-CoV-2); 500 copies/mL(FluA/FluB) | 100 copies/mL(SARS-CoV-2); 500 copies/mL(FluA/FluB) | ||
Clinical Sensitivity | SARS-CoV-2: 100.00% (149/149,95% CI: 97.49% to 100.00%) Flu A: 100.00% (49/49, 95% CI: 92.73% to 100.00%) Flu B: 100.00% (50/50, 95% CI: 92.86% to 100.00%) | SARS-CoV-2: 100.00% (267/267, 95% CI: 98.63-100.00%) Flu A: 100.00% ( 170/170, 95% CI: 98.25-100.00%) Flu B: 100.00% (193/193, 95% CI: 98.46-100.00%) | ||
Clinical Specificity | SARS-CoV-2: 99.34% (150/151, 95% CI: 96.34% to 99.88%) Flu A: 99.60% (250/ 251, 95% CI: 97.78% to 99.93%) Flu B: 100.00% (250/250, 95% Cl: 98.49% to 100.00%) | SARS-CoV-2: 99.75% (807 /809, 95% CI: 99.11-99.97%) Flu A: 100.00% (906/906, 95% CI: 99.67-100.00%) | ||
| Detection Time | 20 mins - 30 mins | |||
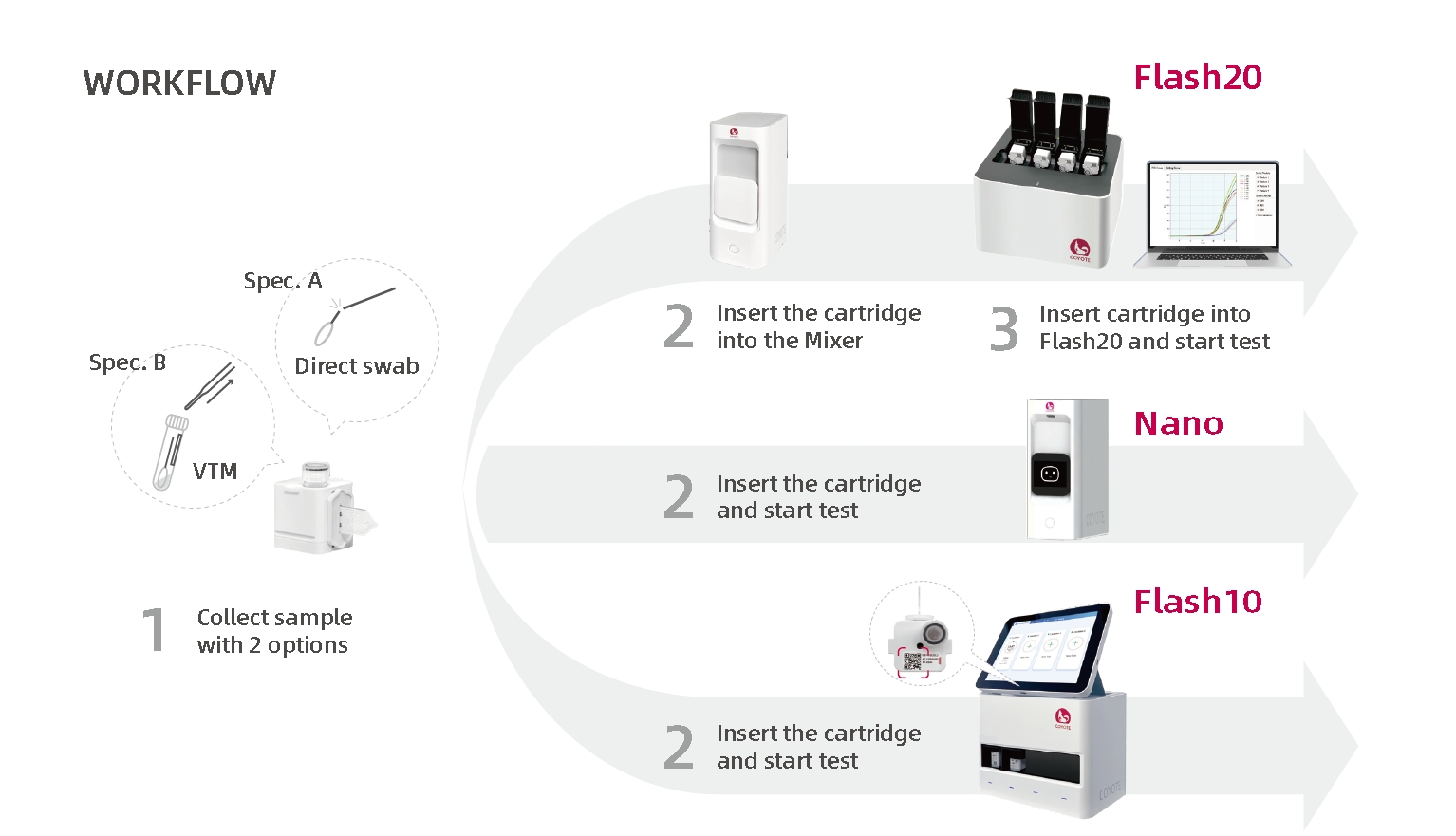
| Sample Type | Nasal, Nasopharyngeal or Oropharyngeal Swabs | |||
| Compatible Instrument | FlashDetect™ Nano, Flash10, Flash20 | FlashDetect™ Nano, Flash10 | ||
| Storage Temperature | 2-28℃ | |||