TB & Respiratory Infections
HAI & Other Infectious Diseases
STIs
Emerging Infectious Diseases
EN
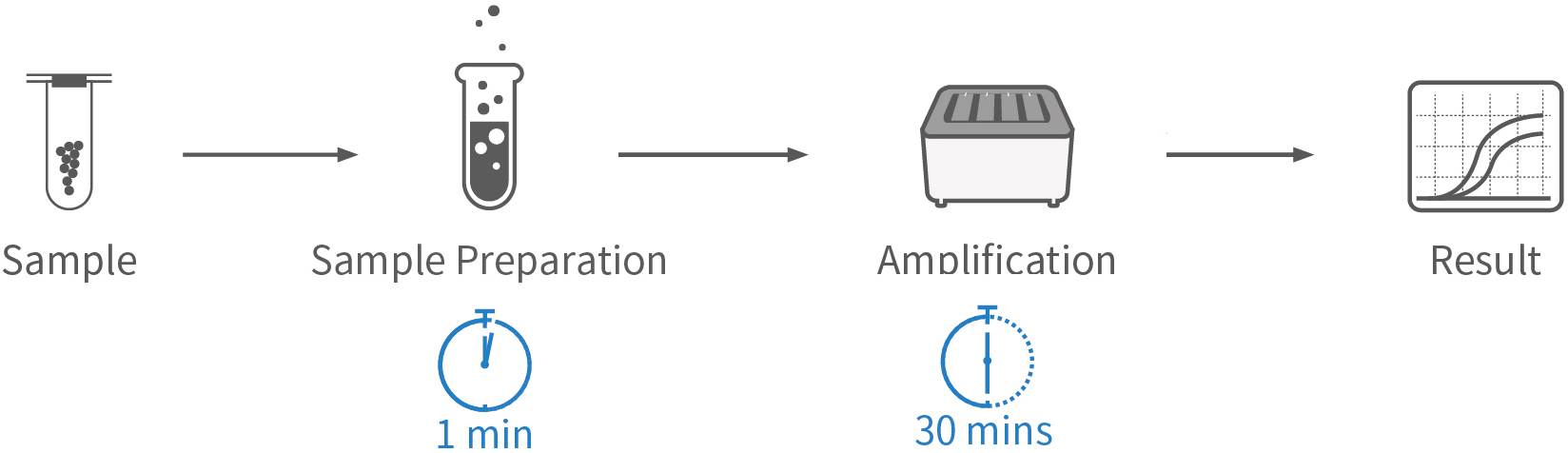
Enable rapid COVID-19 screening by sample preparation within 1 minute, make high-throughput tests available on demand, and provide fast, simple, precise, and high-throughput nucleic acid detection solutions for COVID-19 prevention and control.
Accessible: Easy to operate, saving the efforts of laboratory personnel
Safe: Reduced sample exposure risk for experimenters
Fast: Sample preparation in 1 min and sample-to-result in 30-90 mins
Application: Central laboratory, laboratory department, medical laboratory center, and center for disease control

| Detection Mode | PCR-Fluorescence Probe |
| Sample Type | Nasopharyngeal Swab or Oropharyngeal Swab |
| Sample Prep. Time | 1 min |
| Detection Time | 30 mins (Flash20), 48-90 mins (Others) |
| Sensitivity | ≥ 95% |
| Specificity | ≥ 95% |
| LoD | 500 copies/mL |
| Gene Targets | ORF1ab, N, RNase P |
| Storage Temperature | -20±5℃ |
| Registration | CE-IVD |
Ordering Information
| Cat. No | Pack Size | Compatible System |
| 6018002202/ 06/ 08 -EN | 24/ 48/ 96 tests/kit | Coyote® Flash20 |
| 6018000902/ 06/ 08 -CE | 24/ 48/ 96 tests/kit | Coyote® Mini8 Plus, ABI7500, or Bio-Rad CFX96 |
| 6018001902/ 06/ 08 -EN | 24/ 48/ 96 tests/kit | Coyote® Flash48, ABI7500, or Bio-Rad CFX96 |